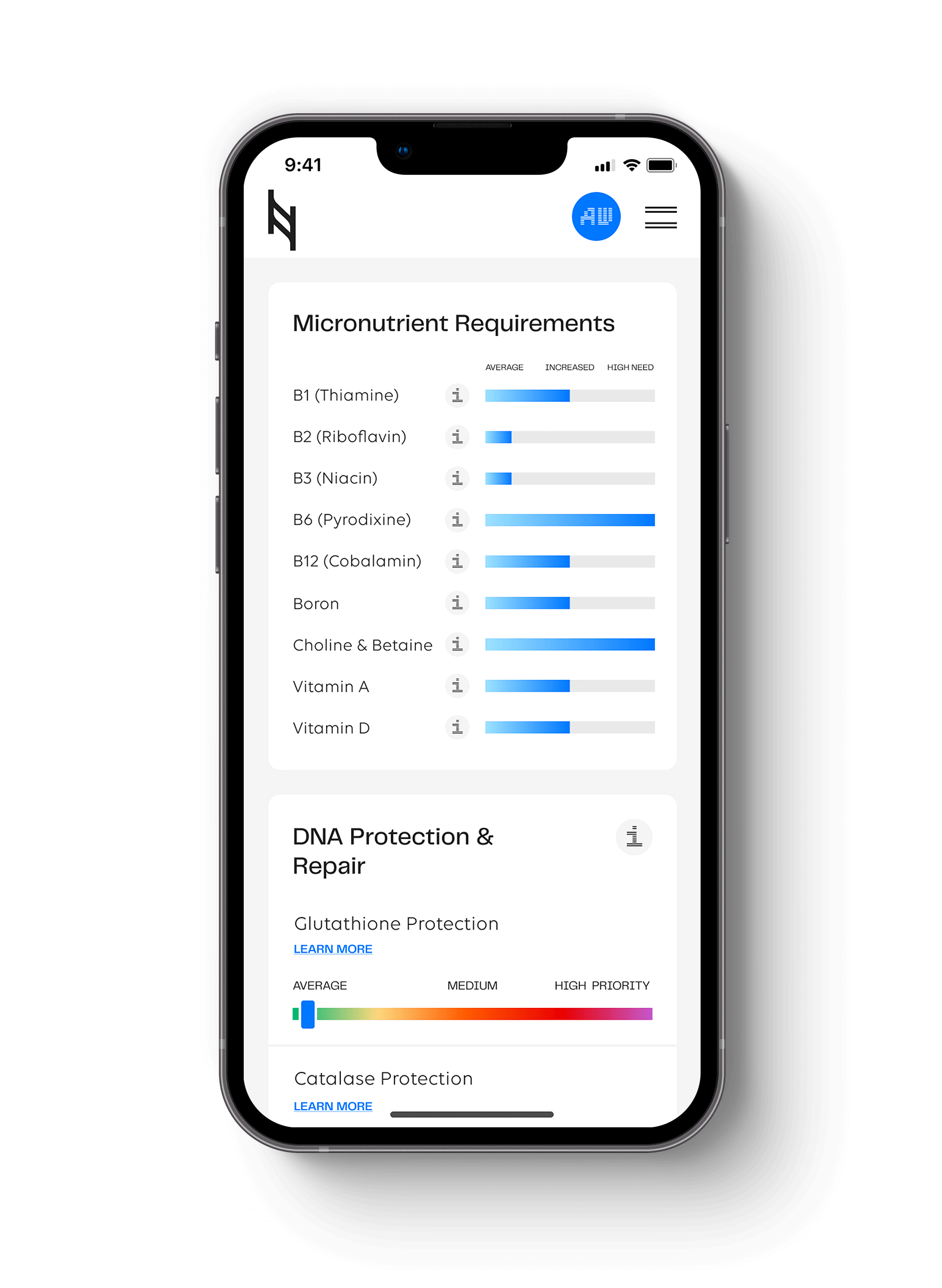
The MTHFR gene stands for methylenetetrahydrofolate reductase. The MTHFR 677 gene produces the MTHFR enzyme that converts methylfolate to 5-MTHF and helps regulate homocysteine levels. Elevated homocysteine has been correlated with inflammation, high blood pressure (certain populations), heart disease, blood clots, depression, macular degeneration, dementia, and cancer.
The reduced enzymatic function of the heterozygous MTHFR 677 is estimated at 30%, and the homozygous MTHFR 677 genotype at 50-70%. Depending on the environment, this increases the need for riboflavin (B2) and folate.
Gene: MTHFR 677 rs1801133
Report Section: Methylation Section
Clinically Significant Genotype: Homozygous AA Genotype
Ancestral Backstory
The highest cluster of the homozygous MTHFR 677 genotype is found in Mexico and Hispanics in the US, Italy, Northern China (Southern China is lower), Spain, and France. The lowest frequency is found in black people (within and outside Africa), Inuit, Finland, Canada, the Netherlands, Germany, and Russia.
Why MTHFR Variants Are More Common in the Mediterranean
One hypothesis is that the homozygous MTHFR genotype was selected based on higher folate intake and UV exposure, both common in Mediterranean climates. What also happens in the body when MTHFR enzymatic function is reduced is that thymidine production increases. Thymidine enhances the repair of UV-induced DNA damage to help quickly repair sun damage.
The sun also depletes folate due to UV radiation. However, darker skin – common in the Mediterranean – contains higher melanin levels, which helps protect against folate loss. Increased thymidine and darker skin protected against the hot sun of the Mediterranean. At the same time, the environment provided more folate-rich fruits and vegetables to supply more dietary folate for other biochemical functions.
As we travel into the colder climates of northern Europe from the Mediterranean, we start to see fewer MTHFR variants. The wild-type becomes the dominant selection due to less UV radiation and lower plant-based folate availability. Along with lighter skin adaptation to northern latitudes for more efficient vitamin D synthesis, the wild-type MTHFR genotype would have been selected to require less folate intake and thymidine production for UV-induced DNA repair.
How Malaria Could Have Shaped MTHFR in Asia
Another hypothesis is that malaria exposure – caused by a parasite through mosquito bites and has been prevalent in the Eastern Mediterranean and Southeast Asia – altered the MTHFR genotype selection.
A study looking at mice infected with malaria was performed with three groups: wild-type MTHFR genotype, heterozygous genotype, and homozygous genotype. The MTHFR homozygous mice had higher T-lymphocytes, natural killer cells, showed protection against malaria, and lived longer than the wild-type.
How is MTHFR connected to this? The malaria parasite needs higher amounts of folate to survive and replicate. Reduced MTHFR function lowered folate levels and boosted levels of thymidine, which may increase lymphocyte replication and immune function in response to malaria. Evolution naturally remedied a situation that antifolate drugs try to mimic. Therefore, in malaria-endemic regions, a homozygous MTHFR 677 genotype becomes advantageous.
Another fascinating connection here is that a gene mutation in alcohol metabolism also occurred in East Asia that causes high levels of acetaldehyde and facial flushing, and one speculation is that the mutation helped kill off parasitic infections and tuberculosis.
Connection to Immunity
For Mediterranean climates, the MTHFR 677 homozygous genotype increases the need for dietary folate. Folate is needed for methylation, which influences glutathione and nitric oxide levels for viral protection. Light skin increases folate requirements further when exposed to more sunshine.
For malaria-endemic regions, a homozygous MTHFR 677 genotype is superior due to lower folate levels. Getting adequate choline, betaine, B6, and B12 would balance out the methylation cycle while providing enhanced protection against malaria.
For viruses, folate is a precursor to BH4 to produce nitric oxide. Nitric oxide acts as an antiviral that is more potent against DNA viruses (HPV, Epstein Barre virus, herpes, and smallpox) in comparison to RNA viruses (COVID-19, measles, Ebola, norovirus, HIV, and polio).
Action Plan
- Check to see if you have the MTHFR 677 AA genotype. If you have a heterozygous MTHFR 677 and MTHFR 1298 gene, this also increases the folate requirement to the equivalent MTHFR 677 homozygous genotype.
- If you do not live in a malaria hotspot, a higher folate intake is recommended for the homozygous MTHFR genotype or heterozygous MTHFR 677 and 1298 combination, especially in warmer climates.
- BH4 is depleted by high blood sugar, high omega-6 intake, chronic stress, high levels of mercury, arsenic, lead and aluminum, aspartame, and oxidative stress. This affects the aggressiveness of DNA viruses.
- To increase BH4, include foods high in folate, vitamin C, L-arginine, B6, magnesium, and selenium.
Sources
1. https://www.ncbi.nlm.nih.gov/books/NBK6561/
2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC384952/
3. https://academic.oup.com/ajcn/article/84/5/1243/4649189
4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4525976/
5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6138472/
6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4835080/
7. https://onlinelibrary.wiley.com/doi/abs/10.1002/humu.22533
8. https://onlinelibrary.wiley.com/doi/full/10.1002/humu.22409
9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4771607/
10. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0143738
11. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0031688
12. https://www.ncbi.nlm.nih.gov/pubmed/9356500
13. https://www.cdc.gov/malaria/about/distribution.html
14. https://onlinelibrary.wiley.com/doi/full/10.1111/jch.13073
15. 5,10-Methylenetetrahydrofolate Reductase Gene Variants and Congenital Anomalies: A HUGE Review
Hit your health goals faster
We'll help you remove the guesswork
Experience the most advanced nutrigenomic test available, covering 100 clinically relevant genes for a "whole body" analysis. Take control of your health today.
$359