Medically reviewed by Dr. Alejandra Carrasco, MD
nourishmedicine.com
Since March of 2020, the research on SARS-CoV-2 and the illness caused by it – COVID-19 – has been getting published at an accelerated rate. Nutrition Genome has been monitoring all of the genetic research on COVID-19, looking for consistency, accuracy, and decipherable patterns around the world. As we begin to see spikes in COVID-19 cases again, we must take action on what we know up until this point.
Our first article in March 2020 examined the hypothesis that SARS-CoV-2 is highjacking the NF-kB pathway and should be a target for suppressing viral aggressiveness. The NF-kB pathway hypothesis was recently validated by Boston University in September 2020.
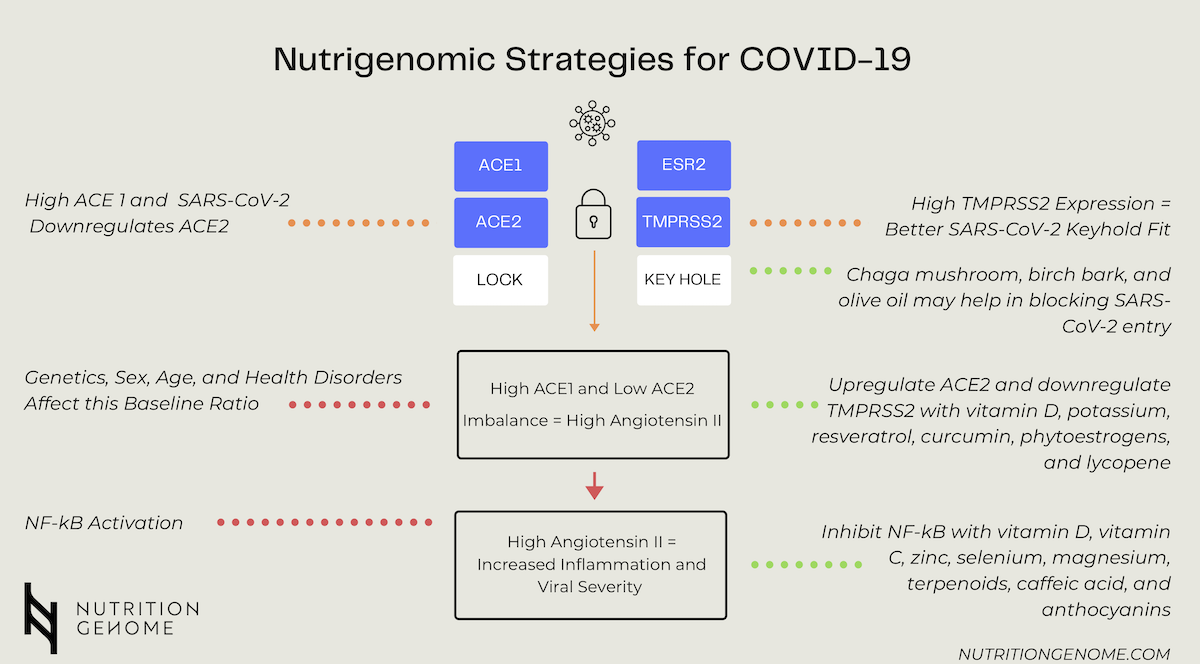
It now appears that there are some genetic insights involving the genotypes and expression of ACE1, AGTR1, ACE2, TMPRSS2, and potentially ESR2 for COVID-19 infection susceptibility and severity. While categorized as a respiratory virus, COVID-19 manifests more like a vascular disease. Up until this point, the majority of research has focused mainly on ACE2. What we are discovering is that it is the combination of ACE1, ACE2, and TMPRSS2.
First, let’s outline what we have learned on how this virus works and why certain people may be more genetically and epigenetically susceptible.
The Role of ACE1, ACE2, and TMPRSS2 in COVID-19
ACE1 is highly expressed in the lung, and ACE2 is mainly clustered in type-II alveolar cells of the lungs, intestine, kidney, and blood vessels. The primary role of ACE1 is the conversion of angiotensin I to angiotensin II, responsible for constricting blood vessels and elevating blood pressure. ACE2 degrades angiotensin II and provides a balance for ACE1 by dilating blood vessels and lowering blood pressure.
Both ACE2 and TMPRSS2 are crucial for SARS-CoV-2 entry into host cells. While ACE2 is the main receptor for the spike protein (coronaviruses are known for their crown of spikes) of both SARS-CoV and SARS-CoV-2, mediating viral attachment to target cells, TMPRSS2 cleaves the spike protein and allows fusion of viral and cellular membranes. This process is similar to viral activation and cell entry of other coronaviruses, including SARS-CoV, and influenza H1N1.
The ACE1/ACE2 baseline ratio, high TMPRSS2 expression, and the down-regulation of ACE2 receptors by SARS-CoV-2 are the key pillars to understand. High ACE1/low ACE2 and increased expression of TMPRSS2 primes the virus for entry. Once the virus fuses with the cellular membranes, it takes control, shuts down more ACE2 receptors, closing the door behind it. Fewer ACE2 receptors lead to elevated angiotensin II levels, which increases the viral load. This leads to a more severe infection, NF-kb activation, lung damage, and viral replication.
There has been conflicting research correlating high or low expression of ACE2 and the susceptibility to COVID-19. There was a past concern with ACE inhibitors increasing ACE2 expression, and therefore, a higher risk for COVID-19 severity. This concern was found to be false and actually, the opposite was true. A Yale study found seniors taking ACE inhibitors had a 40% lower risk of COVID-19 hospitalization. In another study of patients with COVID-19, the viral load and lung injury were strongly associated with the high circulating levels of angiotensin II, caused by the fewer ACE2 receptors. The less ACE2 receptors, the less angiotensin II can be degraded. The biological marker of this imbalance appears to be hypokalemia (low potassium).
Nutrigenomic Research for ACE1, AGTR1, ACE2, TMPRSS2 and ESR2
ACE1
The ACE1 rs4343 gene is characterized by a genetic deletion/insertion (D/I) polymorphism and is associated with approximately 60% of circulating and tissue concentrations of ACE. The GG genotype is associated with a higher baseline of the ACE1 enzyme in serum, AG intermediate, and AA low ACE1. High ACE1 leads to low ACE2.
In a twins study, after six weeks of a high saturated fat diet over 37%, circulating ACE concentrations increased by 15%, accompanied by an increased ACE gene expression in adipose tissue. The wild-type homozygous carriers (GG) of the variant had higher baseline ACE concentrations and additionally showed a 2-fold increase in ACE concentrations in response to the high saturated fat diet as compared to the AA and AG genotype. GG carriers also responded with higher systolic blood pressure as compared to AA and AG carriers.
Women have naturally lower levels of ACE1, and men may have a higher probability of ACE1 expressing higher. The ACE1/ACE2 imbalance during ACE2 receptor suppression in the presence of SARS-CoV-2 infection is more likely in men and may help explain the differences in severity based on sex.
ACE1 inhibition has been demonstrated in research by bilberry, grapes, allicin (raw garlic), cinnamon, and jasmine.
AGTR1
AGTR1 encodes for the Angiotensin-II receptor type 1 enzyme. The CC genotype was also more prone to high blood pressure from a high fat – especially with excessive carbohydrates – diet. The NF-kb pathway is a downstream target of AGTR1. This means that elevated ACE1 and the upregulation of AGTR1 combined puts the body in an inflammatory state, allowing a virus to thrive.
Nitric oxide downregulates AGTR1, balancing out the variant upregulation and blood pressure. To do this, include nitric oxide precursors like L-arginine, folate, vitamin C, magnesium, vitamin D, DHA, and leafy green vegetables.
ACE2
The expression of ACE2 has been detected in nasal epithelial cells, alveolar epithelial type II cells of lungs, and the luminal surface of intestinal epithelial cells. The nose, lungs, and intestine facilitate viral entry and serve as a potential site of viral invasion. However, it appears the expression of TMPRSS2 determines the ability of the virus to enter the cells.
Research has suggested that age, sex, and genetic variants in the ACE2 gene can potentially affect ACE2 levels in the body. Children generally have higher levels of ACE2 than adults. Women have been found to have higher ACE2 levels than men due to the upregulation of ACE2 by estrogen. This could be the foundational reason men and post-menopausal women are more susceptible to COVID-19.
One study of 534 subjects found that 67% of the variation in circulating ACE2 could be accounted for by genetic factors. Among different polymorphisms, it has been speculated that ACE2 rs2106809 might exhibit primary effects on the ACE2 levels. The circulating ACE2 levels tend to be greater in AA or AG genotype compared with that in the GG genotype.
How do you increase ACE2 expression? Potassium, vitamin D, and resveratrol. What was interesting about resveratrol is that it mitigated the effect of a high-fat diet by increasing the expression of ACE2 in animal studies. Betulinic acid (chaga mushroom) and oleanolic acid (olive oil) have also been studied as SARS-CoV-2 entry inhibitors.
TMPRSS2 and ESR2
TMPRSS2 expression is several times higher in the prostate compared with any other tissue, as well as the upper digestive and respiratory tract.
ESR2 is also found in the prostate, lungs, breast, and the cardiovascular system with implications in blood clotting. TMPRSS2 acts like the shape of the keyhole, while ACE2 is the actual lock. A higher expression of TMPRSS2 means a better keyhole fit for viruses. Smoking is one way that promotes a higher expression of TMPRSS2 and increases the risk of SARS-CoV-2 infection.
In a study looking at the Italian population, expression levels of both ACE2 and TMPRSS2 were assessed. Researchers found no significant evidence that ACE2 is associated with COVID-19 severity or sex bias in the Italian population. However, TMPRSS2 levels and genetic variants proved to be possible candidate disease modulators, contributing to the observed epidemiological data among Italian patients. Genes related to higher levels of TMPRSS2 were more frequent in Italians vs. East Asian populations.
The genetic predisposition to severe pH1N1 2009 influenza virus was evaluated in Chinese human subjects, finding that the GG genotype of rs2070788 led to an increased expression of TMPRSS2, a risk variant for a severe pH1N1 influenza infection and it was significantly associated with the susceptibility to IAV H7N9 (Avian flu).
Research has found that the expression of the fusion gene TMPRSS2:ERG, which is thought to be responsible for up to 80% of prostate cancers, is repressed by estrogen receptor beta (ESR2 gene) agonists. Phytoestrogens are agonists known to have up to 30-fold greater affinity for estrogen receptor beta.
Japan has exceptionally low levels of prostate cancer, and the risk rises when their traditional diet changes to a Westernized diet. The Japanese also have some of the lowest death rates from COVID-19 despite having a large elderly population. The traditional Japanese diet contains high levels of dietary phytoestrogens, and since these upregulate estrogen receptor beta and lower the expression of TMPRSS2, and therefore alter the keyhole for SARS-CoV-2 to enter, we may be seeing a nutrigenomic strategy in place that is slowing the virus at the door.
What decreases the expression of TMPRSS2? Phytoestrogens, curcumin, and lycopene (tomato sauce). Research has shown that curcumin binds to receptor-binding domain site of viral S protein and also to the viral attachment sites of ACE2 receptor and downregulates TMPRSS2, demonstrating that curcumin can act as potential inhibitory agent antagonizing the entry of SARS-CoV2 viral protein.
Variants in the ESR2 gene increase the need for phytoestrogens for prostate and breast health. Post-menopausal women will also benefit from increasing phytoestrogen intake.
Strategies
*Please check with your doctor before beginning any supplements or herbs.
- Balance ACE1/ACE2 with appropriate fat intake based on gene results, making sure vitamin D is in range, increasing potassium intake from plants, and foods high in resveratrol. Foods that have demonstrated ACE1 inhibition including bilberry, grapes, raw garlic, cinnamon, and jasmine.
- For those with variants in ESR2 and the TMPRSS2 gene, and post-menopausal women, increase phytoestrogen, turmeric, and lycopene (tomato sauce) intake.
- The best phytoestrogens in research for blood pressure and the ESR2 gene include dark berries (contains resveratrol), beans, rye, hummus, peanuts (contains resveratrol), miso soup, flax seeds (women), tahini sauce, and cruciferous vegetables (broccoli, cabbage, kale, Brussels sprouts).
- Higher levels of TMPRSS2 have been found to upregulate and activate NF-kb creating an inflammatory environment. NF-Kb inhibitors can be both preventative for inflammation and successful with slowing viral aggressiveness. These include vitamin C, vitamin D, zinc, selenium, magnesium, resveratrol (red wine, peanuts, pistachios, blueberries, bilberries, cranberries, cacao, and muscadine grapes), triterpenoids (Chaga, reishi, olive oil, holy basil), caffeic acid (coffee, Chaga, red wine, elderberry), and anthocyanins (elderberry, gogi berries, cacao).
- Zinc and copper should be at a 15mg to 1mg balance. A balanced diet of animal and plant foods typically hit this ratio. A plant-based diet may be low in zinc and high in copper and can cause sodium cravings. High iron levels, malabsorption syndromes, and high amounts of supplemental zinc without copper can push copper too low. Copper deficiency can enhance the vulnerability of the heart and blood vessels to damage.
Other Sources
- https://www.nature.com/articles/s41420-020-0276-1
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7319800/
- https://www.frontiersin.org/articles/10.3389/fped.2020.00206/full
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5865875/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7246956/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6801832/
- https://www.frontiersin.org/articles/10.3389/fphar.2019.01212/full
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7197627/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6612254/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7279323/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7299138/
- https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.120.15353
- https://www.biorxiv.org/content/10.1101/2020.06.10.144964v1.full.pdf